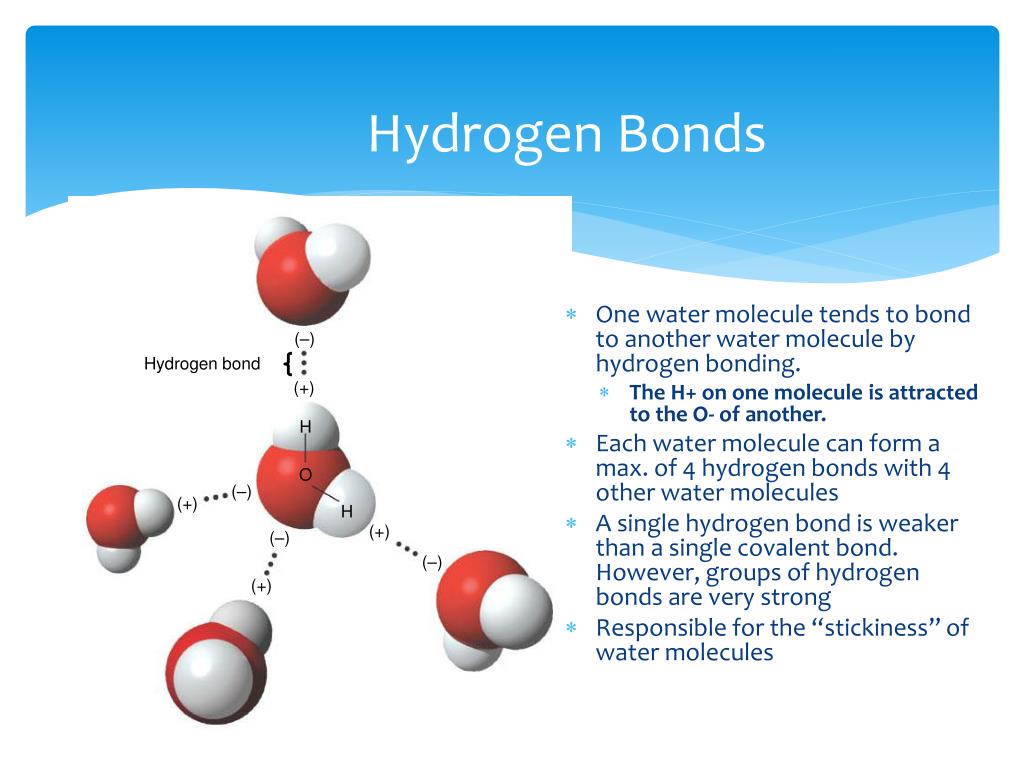
Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding . Web the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. Web identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from. Web a charged or polar substance that interacts with and dissolves in water is said to be hydrophilic: Web polar compounds tend to dissolve in water, and we can extend that generality to the most polar compounds of all—ionic compounds. Web water is called the universal solvent because more substances dissolve in water than in any other chemical. Web the polarity of water and its ability to hydrogen bond contributes to its unique properties. Web water, due to its polarity, acts as a fantastic solvent, especially for polar.
from www.slideserve.com
Web the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. Web polar compounds tend to dissolve in water, and we can extend that generality to the most polar compounds of all—ionic compounds. Web a charged or polar substance that interacts with and dissolves in water is said to be hydrophilic: Web water is called the universal solvent because more substances dissolve in water than in any other chemical. Web the polarity of water and its ability to hydrogen bond contributes to its unique properties. Web identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from. Web water, due to its polarity, acts as a fantastic solvent, especially for polar.
PPT Properties of Water PowerPoint Presentation, free download ID
Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding Web polar compounds tend to dissolve in water, and we can extend that generality to the most polar compounds of all—ionic compounds. Web polar compounds tend to dissolve in water, and we can extend that generality to the most polar compounds of all—ionic compounds. Web the polarity of water and its ability to hydrogen bond contributes to its unique properties. Web water is called the universal solvent because more substances dissolve in water than in any other chemical. Web identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from. Web a charged or polar substance that interacts with and dissolves in water is said to be hydrophilic: Web water, due to its polarity, acts as a fantastic solvent, especially for polar. Web the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the.
From slideplayer.com
Water is the medium of life ppt download Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding Web the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. Web the polarity of water and its ability to hydrogen bond contributes to its unique properties. Web water, due to its polarity, acts as a fantastic solvent, especially for polar. Web identify three special. Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding.
From www.chemistrysteps.com
Solubility of Organic Compounds Chemistry Steps Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding Web water, due to its polarity, acts as a fantastic solvent, especially for polar. Web polar compounds tend to dissolve in water, and we can extend that generality to the most polar compounds of all—ionic compounds. Web water is called the universal solvent because more substances dissolve in water than in any other chemical. Web the polarity of the water. Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding Web identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from. Web a charged or polar substance that interacts with and dissolves in water is said to be hydrophilic: Web the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special. Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding.
From www.slideserve.com
PPT Section 6.3 Water & Solutions PowerPoint Presentation, free Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding Web water, due to its polarity, acts as a fantastic solvent, especially for polar. Web the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. Web identify three special properties of water that make it unusual for a molecule of its size, and explain how. Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding.
From www.ozmo.io
Aqueous Solutions What They Are And How They Work Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding Web the polarity of water and its ability to hydrogen bond contributes to its unique properties. Web water is called the universal solvent because more substances dissolve in water than in any other chemical. Web a charged or polar substance that interacts with and dissolves in water is said to be hydrophilic: Web water, due to its polarity, acts as. Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding.
From rwu.pressbooks.pub
5.1 Properties of Water Introduction to Oceanography Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding Web the polarity of water and its ability to hydrogen bond contributes to its unique properties. Web identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from. Web polar compounds tend to dissolve in water, and we can extend that generality to the most polar compounds of all—ionic. Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding Web identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from. Web the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. Web water, due to its polarity, acts as a fantastic solvent,. Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding.
From studylib.net
Polar and Nonpolar Molecules Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding Web the polarity of water and its ability to hydrogen bond contributes to its unique properties. Web polar compounds tend to dissolve in water, and we can extend that generality to the most polar compounds of all—ionic compounds. Web the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are. Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding.
From geometryofmolecules.com
H2O Polar or Nonpolar Check Covalent bond and polarity Geometry of Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding Web water, due to its polarity, acts as a fantastic solvent, especially for polar. Web identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from. Web water is called the universal solvent because more substances dissolve in water than in any other chemical. Web polar compounds tend to. Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding.
From www.expii.com
Polar vs. Nonpolar Bonds — Overview & Examples Expii Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding Web water is called the universal solvent because more substances dissolve in water than in any other chemical. Web the polarity of water and its ability to hydrogen bond contributes to its unique properties. Web the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the.. Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding.
From quizlet.com
hydrogen bond between water molecules Diagram Quizlet Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding Web the polarity of water and its ability to hydrogen bond contributes to its unique properties. Web water is called the universal solvent because more substances dissolve in water than in any other chemical. Web water, due to its polarity, acts as a fantastic solvent, especially for polar. Web polar compounds tend to dissolve in water, and we can extend. Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding.
From saylordotorg.github.io
Aqueous Solutions Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding Web a charged or polar substance that interacts with and dissolves in water is said to be hydrophilic: Web water is called the universal solvent because more substances dissolve in water than in any other chemical. Web identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from. Web. Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding.
From slideplayer.com
Water and Life Processes ppt download Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding Web water, due to its polarity, acts as a fantastic solvent, especially for polar. Web identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from. Web water is called the universal solvent because more substances dissolve in water than in any other chemical. Web the polarity of the. Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding.
From ar.inspiredpencil.com
Properties Of Water Polarity Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding Web a charged or polar substance that interacts with and dissolves in water is said to be hydrophilic: Web identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from. Web water, due to its polarity, acts as a fantastic solvent, especially for polar. Web the polarity of water. Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding.
From www.britannica.com
covalent bond Definition, Properties, Examples, & Facts Britannica Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding Web polar compounds tend to dissolve in water, and we can extend that generality to the most polar compounds of all—ionic compounds. Web water, due to its polarity, acts as a fantastic solvent, especially for polar. Web a charged or polar substance that interacts with and dissolves in water is said to be hydrophilic: Web the polarity of the water. Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding.
From www.youtube.com
Polarity of Water and Hydrogen Bonds (2016) IB Biology YouTube Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding Web the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the. Web water, due to its polarity, acts as a fantastic solvent, especially for polar. Web a charged or polar substance that interacts with and dissolves in water is said to be hydrophilic: Web water. Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding.
From slideplayer.com
Water and the Fitness of the Environment ppt download Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding Web water is called the universal solvent because more substances dissolve in water than in any other chemical. Web identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from. Web the polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special. Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding.
From slideplayer.com
Water and Life Processes ppt download Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding Web identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from. Web water, due to its polarity, acts as a fantastic solvent, especially for polar. Web a charged or polar substance that interacts with and dissolves in water is said to be hydrophilic: Web the polarity of the. Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Bonding.